CBS News Live
CBS News Miami: Local News, Weather & More
CBS News Miami is your streaming home for breaking news, weather, traffic and sports for the Miami area and beyond. Watch 24/7.
Watch CBS News
CBS News Miami is your streaming home for breaking news, weather, traffic and sports for the Miami area and beyond. Watch 24/7.





The series moves to Miami for Games 3 and 4 on Saturday and Monday.

Hialeah police say teens on a joyride ended up involved in a crash that killed two women and left another critically injured.

On Wednesday, 30-year-old Jose Hernandez, a former Miramar police officer, pled guilty to a first-degree felony of aggravated child abuse.

The Dolphins have a first-round draft pick for the first time since 2021

The grass fire which burned 20 acres along the Turnpike in SW Miami-Dade had been mostly contained by Monday evening.

It looks like a regular gravel road on top of a levy, but underneath the stone is a huge "seepage wall." It's meant to keep water from the eastern edge of the Everglades National Park from seeping out and flooding nearby properties.

Entering its third year, Miami has been chosen as one of the only six venues to host an F1 Sprint in 2024, meaning there will be an extra race held that Saturday and guaranteeing high-octane action throughout the weekend.

Miami Beach police have a new eye in the sky, which is the X10 drone.

How did you bracket do against our VIPs

The innovative program launched at JC Bermudez Doral Senior High School is making Miami Proud

The challenges of growing up without a father figure are experienced every day by young women

Plastic and reconstructive surgeon Dr. Joshua Lampert will host a charity surgery day in honor of his mother who very instrumental in his life

Case is a successful businesswoman, generous philanthropist, mentor, and 2024 TIME Dealer of the Year

"It's a good day for America, it's a good day for Europe and it's a good day for world peace," Mr. Biden said in remarks from the White House.

The outcome of the immunity case before the Supreme Court will have significant ramifications for former President Donald Trump's federal criminal prosecution in Washington, D.C.

The USDA had floated banning flavored milk options from some school lunches.

A Texas grand jury indicted more than 140 migrants on misdemeanor rioting charges over an alleged mass attempt to breach the U.S.-Mexico border, a day after a judge threw out the cases.

The series moves to Miami for Games 3 and 4 on Saturday and Monday.
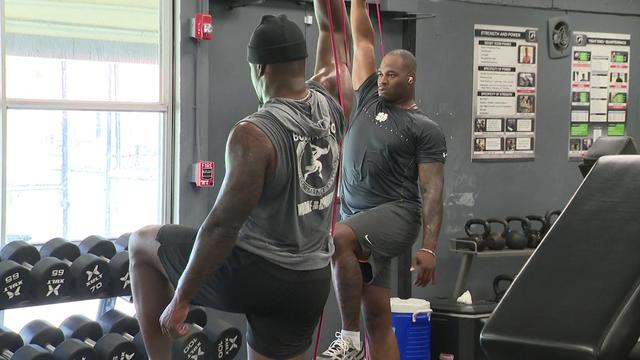
From current players in the league, like Miami Dolphins wide receiver Tyreek Hill, to guys hoping to get their name called in this year's draft, local trainer Pete Bommarito has seen it all.

In the moment, The Save kept the game tied.

Carter Verhaeghe lifted a backhander just under the crossbar 2:59 into overtime, and the Florida Panthers beat the Tampa Bay Lightning 3-2 for a 2-0 lead in their NHL first-round playoff series.

Sports agents work overtime to get their clients the best deal and take care of them

Max Fried pitched a three-hitter for Atlanta's first nine-inning complete game since 2022, Adam Duvall had a two-run homer and the Atlanta Braves blanked the Miami Marlins for the second night in a row, 5-0.

CBS News Miami will bring you coverage of the Miami Dolphins draft like no one else can

Watch CBS News Miami's special live coverage of the NFL draft Thursday at 7 p.m.

Travis d'Arnaud hit his fifth home run in four games and Bryce Elder pitched 6 2/3 spotless innings in his return from the minors as the Atlanta Braves beat the Miami Marlins 3-0.
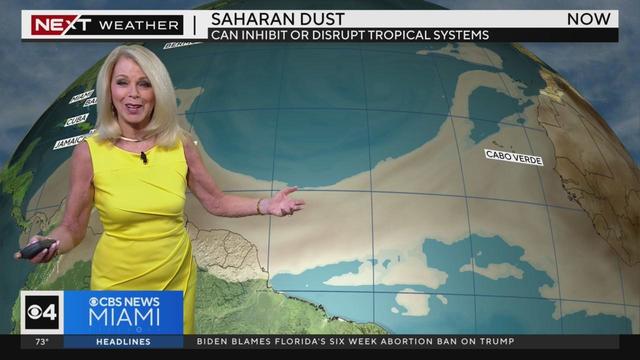
CBS News Miami meteorologist Cindy Preszler's weather outlook for South Florida.
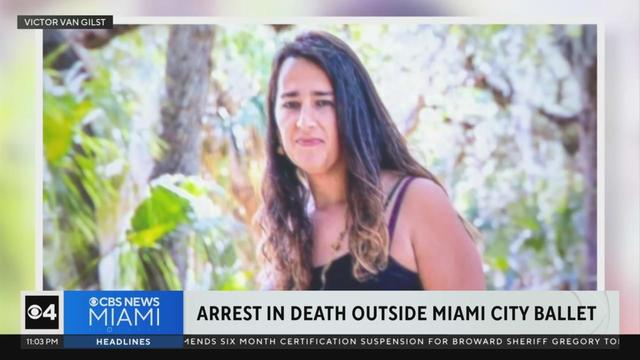
An arrest has been made after the body of a woman, who was brutally beaten in front of the Miami City Ballet on Miami Beach, was discovered early Tuesday morning.

Hialeah police say teens on a joyride ended up involved in a crash that killed two women and left another critically injured.

Hialeah police say teens on a joyride ended up involved in a crash that killed two women and left another critically injured.

An arrest has been made after the body of a woman, who was brutally beaten in front of the Miami City Ballet on Miami Beach, was discovered early Tuesday morning.

The series moves to Miami for Games 3 and 4 on Saturday and Monday.

Hialeah police say teens on a joyride ended up involved in a crash that killed two women and left another critically injured.

On Wednesday, 30-year-old Jose Hernandez, a former Miramar police officer, pled guilty to a first-degree felony of aggravated child abuse.

The Dolphins have a first-round draft pick for the first time since 2021

The grass fire which burned 20 acres along the Turnpike in SW Miami-Dade had been mostly contained by Monday evening.

Florida insurance companies made money last year for the first time in seven years.

The U.S. Supreme Court is scheduled Sept. 26 to discuss whether it will hear a First Amendment challenge to a 2021 Florida law that placed restrictions on major social-media companies.

The number of people traveling to Florida during the second quarter of 2023 decreased compared to a year earlier, according to estimates released Wednesday by the state's tourism-marketing agency.

Gov. Ron DeSantis on Monday said Walt Disney Parks and Resorts should drop a federal lawsuit that claims retaliation by the state and accept changes to a special district that long benefited the theme-park giant.

It's Back-To-School and that also means back to the lunch room, as breakfast and lunch are free to all Miami-Dade public school students again this year.

"It's a good day for America, it's a good day for Europe and it's a good day for world peace," Mr. Biden said in remarks from the White House.

The outcome of the immunity case before the Supreme Court will have significant ramifications for former President Donald Trump's federal criminal prosecution in Washington, D.C.

The USDA had floated banning flavored milk options from some school lunches.

A Texas grand jury indicted more than 140 migrants on misdemeanor rioting charges over an alleged mass attempt to breach the U.S.-Mexico border, a day after a judge threw out the cases.

Lawmakers argue the Chinese government can use the widely popular video-sharing app as a spy tool and to covertly influence the U.S. public.

Jim DeFede talks to former state senator Jeff Brandes, who runs the Florida Policy Project, which has been exploring solutions that include the role of vouchers and something called "upzoning."

Jim DeFede continues scrutinizing the three-plus decades during which Katherine Fernandez Rundle has led the Miami-Dade State Attorney's Office. This week, Jim's guest is South Miami Mayor Javier Fernandez.

Jim DeFede talks to the CEO of Trulieve, the cannabis company that spent $40 million to gather the signatures to get the measure on the ballot.

Jim DeFede goes one-on-one with the political and community activist and former rap superstar, who talks about making a run for the Rep. Sheila Cherfilus-McCormick's seat.

CBS News Miami's Jim DeFede speaks to attorneys about Rundle's tenure as Miami-Dade State Attorney.

Federal officials say they're double checking whether pasteurization has eradicated the danger from possible bird virus particles in milk.

UnitedHealth said it paid the criminals behind attack that crippled hospitals and pharmacies to protect sensitive patient data.

The CDC estimates the U.S. could reach 300 measles cases in 2024 — more than the recent peak two years ago.

Health officials are warning consumers not to consume Infinite Herbs basil sold at some Trader Joe's and Dierberg's stores after 12 people were sickened.

Organic option is best when buying certain produce, especially blueberries, nonprofit group says in analysis of chemical residues.

These best cooling floor fans from Dyson, Honeywell, Black+Decker and more will cool you in the months ahead.

The best meal kits for weight loss make it easy (and fun!) to stick to a healthy, consistent diet.

I sampled America's most popular meal kit to see how it stacks up in terms of price, accessibility, and more.

Save money on a new hearing aid with the top budget-friendly hearing aids of 2024.

If you aren't interested in picking up the newest iPhone model, these phones are all great in their own right.

Has mom and dad's bank been open too long at your house?

One consumer reported suffering a dental injury after eating Trader Joe's Chicken, Lentil & Caramelized Onion Pilaf product.

The company will keep its name and headquarters in Pittsburgh, but the United Steelworkers union voiced opposition to the deal.
For customers of SmileDirectClub, they were greeted with a message that they had shut down when logging on to its website.

CBP officials say the individually wrapped pink packages have an estimated resale value of more than $19,000.

For the first time in more than a year, the monthly board meeting of Walt Disney World's governing district was back to being what many municipal government forums often are — boring.

Taylor Swift broke her own records, Spotify said, and now owns the record for the top three most-streamed albums in a single day.

The singer was found deceased at her home, a representative said.

Anticipation was growing at a fever pitch before Taylor Swift's latest album, "The Tortured Poets Department," dropped at midnight EDT. But it turned out it's actually a double album.

Guitar legend Dickey Betts, who co-founded the Allman Brothers Band and wrote their biggest hit, "Ramblin' Man," has died.