
FDA approves new nasal spray to treat migraine headaches in adults, Pfizer says
The U.S. Food and Drug Administration approved a new nasal spray as a rapid treatment for migraine pain in adults.
Watch CBS News

The U.S. Food and Drug Administration approved a new nasal spray as a rapid treatment for migraine pain in adults.

Pfizer's updated COVID-19 booster significantly revved up adults' virus-fighting antibodies, the company said Friday, releasing early findings from a rigorous study of the new shots.
The federal budget for buying and distributing COVID-19 vaccines will run out "as early as January."

Federal health officials say thousands of updated booster shots are already being shipped around the country.

Florida is the only state in the country without plans to preorder COVID vaccines for children as young as 6 months old, following the FDA's decision to approve both Pfizer and Moderna's vaccines.

The White House says doses will be available everywhere from pediatricians' offices to children's museums, if health authorities sign off on the shots later this month.

Kids younger than 5 are one step closer to being eligible for a COVID-19 vaccine. Pfizer applied for emergency use authorization for kids 6 months to 5 years old -- the only age group vaccines are not yet approved for. Nikki Battiste reports.

It is meant to help prevent severe illness and death from COVID-19. But researchers say people using who have a COVID relapse may be able to spread COVID without even knowing it.

Researchers say Paxlovid users who have a COVID relapse may be able to spread the virus without even knowing it.
There haven't been reports of anything happening to people who've taken this medication, but Pfizer is recalling it just in case.

The Food and Drug Administration announced a big step in the fight against the pandemic.

Pfizer announced new results on Tuesday from studies testing its five-day course of COVID antiviral pills, confirming Paxlovid cut the risk of hospitalization or death in a trial for patients at high-risk of severe COVID-19 by 89% when treated within the first three days of their symptoms.
The Food and Drug Administration has greenlighted a request from Pfizer and BioNTech to allow Americans as young as 16 to get a booster shot of their COVID-19 vaccine, the agency announced on Thursday, clearing a key hurdle before that age group can receive the third shot. Boosters were previously OK'd for ages 18 and up.
Pfizer's booster shot may be available to all adults in America as early as this weekend.
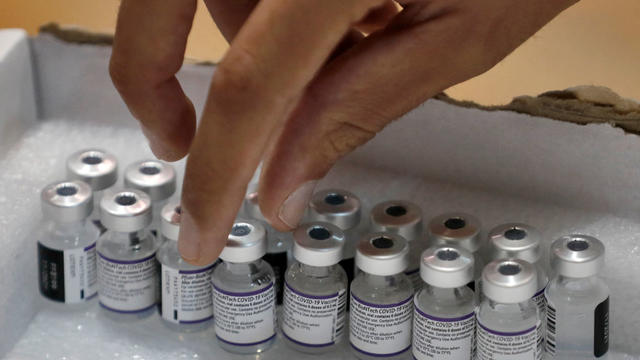
The company says new data from a large clinical trial of more than 10,000 fully vaccinated people found that a booster dose was over 95% effective against COVID-19 compared to individuals who were fully vaccinated but had not received a booster dose.
The CDC just approved the COVID vaccine for younger children, but that may leave adults wondering what is next for them.

The CDC has given its final okay for about 28 million grade school children to get Pfizer's COVID-19 vaccine.
The Pfizer COVID-19 vaccine has officially been authorized for children ages 5-11. This comes after a sign-off by the CDC Tuesday night.

The Food and Drug Administration authorized a smaller dose of Pfizer's COVID-19 vaccine for children ages 5 to 11, a critical step for the more than 28 million children that may soon be eligible.

Pfizer's request to roll out COVID-19 vaccines for Americans as young as 5 years old cleared a key regulatory hurdle Tuesday, after a panel of the Food and Drug Administration's outside vaccine advisers voted by a majority to back Pfizer's request.

The Chief Medical Officer for Joe DiMaggio Children's Hospital says the new proposed Pfizer vaccine for children between the ages of 5 and 11 could be a "lifesaver."
With the FDA considering a Pfizer request to allow vaccination for kids 5 to 11, health experts are seeing that as some very positive news.

The Jackson Health System is now offering a 3rd dose of the Pfizer COVID-19 vaccine to the 13,000 employees of its 7 hospitals.

A panel of the Centers for Disease Control and Prevention's outside vaccine experts voted Thursday to allow use of booster shots for many adults first vaccinated with Pfizer's COVID-19 vaccine at least six months ago, including those 65 and over and others at higher risk of severe COVID-19, clearing one of the last regulatory hurdles for third doses to be given this week.
The authorization makes the Biden administration's plan to roll out booster shots this week at least partially possible.

Indira and Alex Ortega said they lost everything

"You're not a lottery winner, you're a criminal," said the sheriff.

Miami-Dade Animal Services hosting the event on Saturday

It's April 25th at the Amerant Bank Arena in Sunrise

Starbucks unveiled the new cups ahead of Earth Day and as a new report warns plastic production emissions are even greater than those from aviation.

Indira and Alex Ortega said they lost everything

"You're not a lottery winner, you're a criminal," said the sheriff.

Miami-Dade Animal Services hosting the event on Saturday

It's April 25th at the Amerant Bank Arena in Sunrise

Starbucks unveiled the new cups ahead of Earth Day and as a new report warns plastic production emissions are even greater than those from aviation.

Florida insurance companies made money last year for the first time in seven years.

The U.S. Supreme Court is scheduled Sept. 26 to discuss whether it will hear a First Amendment challenge to a 2021 Florida law that placed restrictions on major social-media companies.

The number of people traveling to Florida during the second quarter of 2023 decreased compared to a year earlier, according to estimates released Wednesday by the state's tourism-marketing agency.

Gov. Ron DeSantis on Monday said Walt Disney Parks and Resorts should drop a federal lawsuit that claims retaliation by the state and accept changes to a special district that long benefited the theme-park giant.

It's Back-To-School and that also means back to the lunch room, as breakfast and lunch are free to all Miami-Dade public school students again this year.

Two U.S. officials tell CBS News an Israeli missile has hit Iran in apparent retaliation for the recent drone and missile attack on the Jewish state.

Cuba's deputy foreign minister tells CBS News that his country is willing to accommodate more than one deportation flight per month.

Details of his trip to Tampa have not been released

Pro-Palestinian demonstrators have reoccupied Columbia University's main lawn for a third day of protests in Upper Manhattan.

House Speaker Mike Johnson is including the TikTok divest-or-ban bill in an aid package for Ukraine and Israel.

Jim DeFede talks to the CEO of Trulieve, the cannabis company that spent $40 million to gather the signatures to get the measure on the ballot.

Jim DeFede goes one-on-one with the political and community activist and former rap superstar, who talks about making a run for the Rep. Sheila Cherfilus-McCormick's seat.

CBS News Miami's Jim DeFede speaks to attorneys about Rundle's tenure as Miami-Dade State Attorney.

CBS News Miami's Jim DeFede talks to State Senator Lauren Book about what the Florida Supreme Court has to say about the subject.
Jim DeFede speaks with the head of Florida Decides Healthcare, who is working to get a constitutional amendment to expand Medicaid on the ballot in 2026.

Health officials are warning consumers not to consume Infinite Herbs basil sold at some Trader Joe's and Dierberg's stores after 12 people were sickened.

Organic option is best when buying certain produce, especially blueberries, nonprofit group says in analysis of chemical residues.

The $872 million most likely excludes any amount UnitedHealth may have paid to hackers in ransom.

George Schappell and sister Lori, of Reading, Pa., were the world's oldest conjoined twins, according to the Guinness Book of World Records.

Most worrisome gaps involve cancer chemotherapy drugs, ER medications and and therapies for ADHD.

Find out how and when to watch the US Open Pickleball Championships, including who's competing.

Find that special someone by signing up for one of the undeniably best dating apps in 2024.

Give your grad the best of the best for downtime enjoying music, listening to podcasts, or watching movies and TV.

Discover the best smart grills that make your outdoor grilling easier and more fool-proof than ever.

From Hey Dude shoes to a luxury toilet upgrade, Sam's Club shoppers are going wild for these trending products.

Has mom and dad's bank been open too long at your house?

One consumer reported suffering a dental injury after eating Trader Joe's Chicken, Lentil & Caramelized Onion Pilaf product.

The company will keep its name and headquarters in Pittsburgh, but the United Steelworkers union voiced opposition to the deal.
For customers of SmileDirectClub, they were greeted with a message that they had shut down when logging on to its website.

CBP officials say the individually wrapped pink packages have an estimated resale value of more than $19,000.

Anticipation was growing at a fever pitch before Taylor Swift's latest album, "The Tortured Poets Department," dropped at midnight EDT. But it turned out it's actually a double album.

Guitar legend Dickey Betts, who co-founded the Allman Brothers Band and wrote their biggest hit, "Ramblin' Man," has died.

The 2024 Latin Grammys will return home to Miami — where the organization is headquartered.

O.J. Simpson's longtime lawyer in Las Vegas says the end came quickly.

The new single, titled "Primrose Hill", was recently released by James McCartney and Sean Ono Lennon, who are both musicians themselves.